Reference Potential
In electrochemistry and electrochemical biosensors there is always a reference electrode against which we measure the potential/voltage at the working electrode. For example when we talk about a voltage of 0 V we should more accurately say 0 V vs. a reference. The reference electrodes commonly used in laboratory studies is often a silver/silver chloride electrode/3 M chloride, which is constructed from a silver wire coated with silver chloride in a 3 molar chloride solution.
Please see the button below for a calculator for converting between reference electrodes.
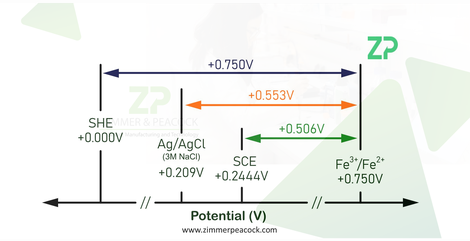
Below are two calculators for calculating the potential of a silver/silver chloride reference electrode versus a standard hydrogen electrode (SHE), based on either concentration of chloride or activity of chloride; for most practical applications we recommend the concentration calculator.