On this page we describe the cost to achieve regulatory approval and the technology stack required to bring an immuno-type biosensor to market, when using a gold electrode as the working electrode of the biosensor
INTRODUCTION
Many groups that use a recognition element such as an: aptamer, antibody, ssDNA, receptors etc, use a gold working electrode, this is because the self assembled molecular layer (SAM) chemistry where a thiol group (SH) is tethered to a gold electrode (Au) is a robust tactic for functionalizing electrodes into electrodes with specificity to the analyte of interest.
PRIOR ART
Zimmer and Peacock is an ISO13485 contract developer and manufacturer of electrochemical biosensors and are well versed in functionalizing our Au-303 electrodes into biosensors, using SAM chemistry. An example of this type of work is shown in Figure 1.0.0, where the Au-303 electrode has been functionalised with a macromolecule. The binding of the subsequent protein analyte is subsequently detected using impedance spectroscopy.
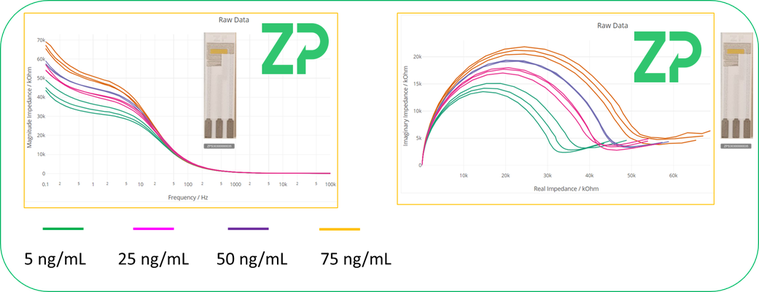
Figure 1.0.0
ROADMAP
For those who have a plan to go to market with gold electrodes functionalized in an immunosensor configuration then ZP has a roadmap. The strategy at ZP is not to treat the gold electrode in isolation of the total system. At ZP we see that the gold electrode is just a single part of the technology as we see the total system is a combination of: electrode, functionalization chemistry, microfluidics, electronics, mechanical, electrochemistry, data science, regulatory affairs, quality management system (QMS), clinical support, etc.
One of the first steps at ZP is to plan out the total plan to get to regulatory approval and market, we call this process Client Onboarding.
ZP Accelerator
One of the first steps with ZP is to get out of the lab and start testing real samples on our ZP Accelerator platform. Many developers, entrepreneurs and start-ups think that they need perfect products in order to start to gather pre-clinical data, at ZP our philosophy is that it is best to get the sensors into the real-world and start testing real samples- we call this the ZP Accelerator.
Regulatory Approval
For an IVD the most common route to market is a 510k or a PMA, these are discussed in our regulatory article.
