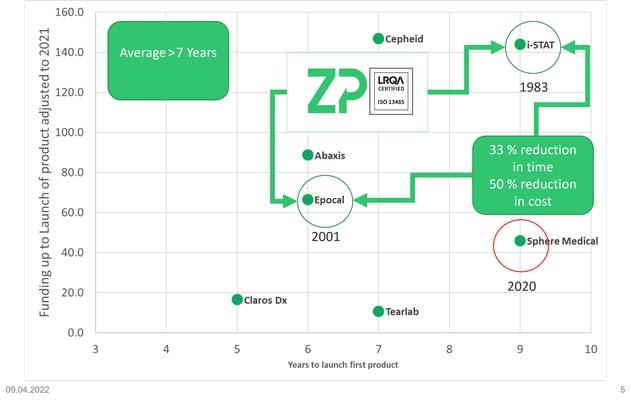
At ZP we perform contract development and manufacturing of electrochemical biosensors, one of the questions we are regularly asked is 'what does it cost to bring medical diagnostic onto the market?'
In this video we use examples of Cepheid, i-STAT, Epocal, Abaxis, Claros Dx, Tearlab, Sphere Medical and now Cue Health to illustrate the effort and cost to bring an in-vitro diagnostic (IVD) to market.
Zimmer and Peacock provides regulatory affairs and quality assurance (RAQA) across the medical devices and diagnostics industry.
In a recent webinar we discussed the 510k route versus the PMA route when seeking regulatory approval in the USA.
ARTICLE FROM DIACEUTICS
The original article is here - click here.
In the adjacent table we have read and understood the information from Diaceutics and added our own take on their numbers.
You can see that Diaceutics gave a value in the range $20 M to $100 M.
CONCLUSION
At ZP we are always excited to work with clients who are ambitious and are driving to the market, and of course we want to support people on this road. We hope that these notes will help people in their buisness planning and their buisness models.
If you have questions regarding ZP's contract development and manufacturing services for IVD, please don't hesitate to contact us.