At ZP we are often asked what is the accuracy of the ZP electrochemical sensors and biosensors, but the answer to the question is 'how do you intend to use them?'. The accuracy of an electrochemical biosensor is a function of the sensor, your sample and your system in which you place the sensor.
The second question is how will your sensor work in my fluid, and these can range from: urine, blood, plasma, serum, saliva, breath, foods, beverages etc, a sub-question to this question is what will the drift be like. Again the answer to this questions is as above, and the way the sensor will respond to your sample depends on your sample and the degree of drift with time depends on the sensor, your sample and your system. In this note we do give a calibration routine that will allow you to compensate for drift and keep the overall system accurate.
Though this note is written with the ZP pH sensors specifically in mind the equations/similar equations and calibration strategies are applicable to many of our other potentiometric sensors including: nitrate, ammonium, phosphate, sodium, potassium, chloride and calcium. At the same time similar equations and calibration routines are applicable to our amperometric sensors: glucose, oxygen, lactate and nitric oxide sensors.
As stated above this note is specifically focused on pH sensors, and the problem that we are trying to solve is drift in pH sensors which are expected to be in continuous use for hours/days.
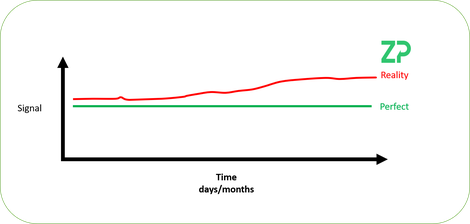
We have illustrated the problem in the adjacent figure, where a pH sensor is in a constant pH solution at a constant temperature and the signal is stable/perfect. The reality is that the signal can/does drift. In this note we explain the maths behind the senors and where the source of drift could be coming from and then calibration routines to counter the drift.
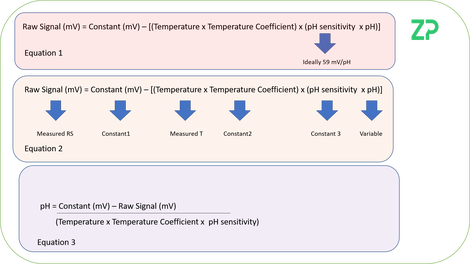
In the adjacent image we have started with an equation based on the Nernst Equation, Equation 1, and rearranged it to Equation 3. If we look at Equation 2 it says that the raw signal will be constant when pH, temperature, constant 1 constant 2 and constant 3 are all stable. What Equation 3 indicates is that to remove the influence of temperature from the signal either the analysis should be done at a controlled temperature or the sample temperature should be monitored.
The reality with pH sensors is that constant 1, constant 2 and constant 3 are not stable, and so if you have wondered why there is always/often bottles of pH calibration solutions next to the lab pH meter it is because of the drifts in these constants; in the rest of the note we discuss re-calibration techniques.
ONE POINT CALIBRATION
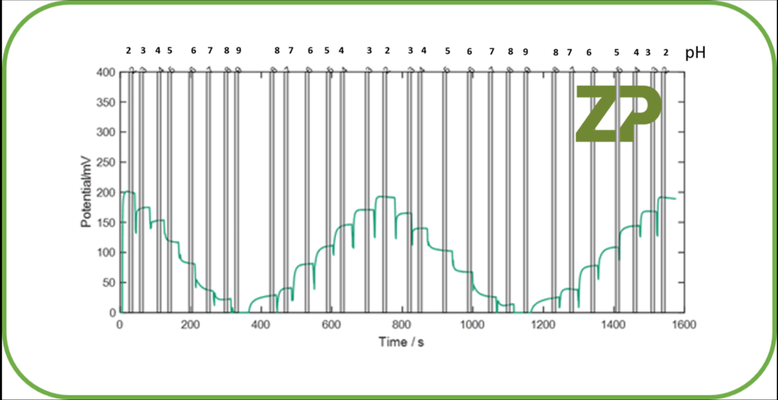
If it is only the Constant 1 in Equation 3 that is drifting then a one point calibration is sufficient. What we mean by this is that a solution of known pH is placed in contact with the sensor and the Constant can be calculated from Equation 4. The now re-calculated constant can be put back into Equation 3 and the accuracy of the system improves and the drift in the raw signal will not be reflected in the displayed pH. In the adjacent image we show raw data from a ZP pH sensor, the steps in the data reflect the changes in signal as the pH is changed from pH 2 to 9 and back again. It should be noted that the sigan is stable over 1600 seconds/30 minutes. We would therefore suggest that the ZP sensors are at least stable over 30 minutes and shouldn't require calibration in that time. Please note a one point calibration is most effective when measuring a sample that should be at a specific pH, for example pH 7.2. In this case one uses a calibration solution that is similar to the sample solution and is also buffered at pH 7.2
TWO POINT CALIBRATION
A one point calibration described above is fine if we assume that the drifting is due to the Constant 1 in Equation 2, but is due to the pH Sensitivity drifting then we need to perform a two point calibration. In the embedded excel file opposite we have embedded the maths to do a two point calibration. To make the spreadsheet work, then the user/system needs to expose the sensor to a calibration solution at pH 4 and record the mV signal (the default in the spreadsheet is 150 mV), next the user/system needs to expose to pH 10 (the default in the spreadsheet is pH -60), from that the spreadsheet calculates the Constant 1 and the pH Sensitivity. These values can be placed back into Equation 4 and the overall system will then calculate the pH more accurately. Typical values used to calibrate a pH sensor are 4 and 10 but the best way to get an accurate calibration is to use calibration solutions whose pH values are reflective of the expected pH range of the solution.
HARDWARE SETUP FOR RECALIBRATION
In the adjacent image we show an example of a hardware setup that can be used for one point and two point calibration.
TEMPERATURE COMPENSATION
The topic we have not yet discussed in this note is temperature compensation, please note that this page is a live page and ZP are busy gathering the temperature characteristics of the pH sensor.