At ZP we are aware that we have 'too many' biosensors, and as the demands of our clients increase and as many approach the market then we have moved to improve the sensors. In this note we discuss that the sodium sensors have been improved in two important ways:
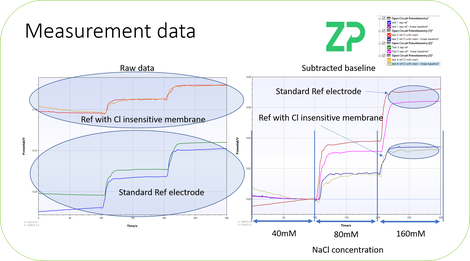
1) CHLORIDE RESILIENT - We now have a sodium sensor that is chloride resilient, what we meant by this is that the reference electrode does not change it's voltage as the chloride concentration changes. In many blood, plasma and serum applications the chloride concentration is fairly fixed at around 150 mM and so the fact that the silver/silver chloride reference electrode is chloride sensitive doesn't matter, but in other fluids this is not the case. In the new sensor the reference electrode is chloride resilient and so suitable for applications where chloride is variable or unknown
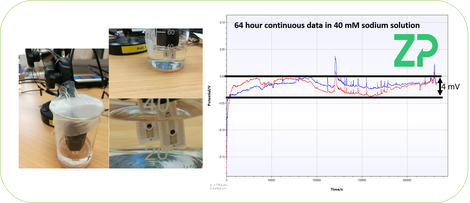
2) LONGER TERM STABILITY - In the adjacent image ZP has run two of its chloride resilient sodium sensors in 40 mM sodium solution for 64 hours. Both sensors kept within a 40 mV wide band. This would mean that the sensor was showing a change that was equivalent to going from 40mM to approximately 160 mM. Both sensors were on the same solution but were in fact run on separate instruments. This would make one think that something extrinsic to the sensor and the solutions was causing the solutions/sensior to change its apparent sodium concentration; an obvious parameter would be temperature. One would conclude that if we repeated the experiment but with temperature control then the apparent stability would be improved.
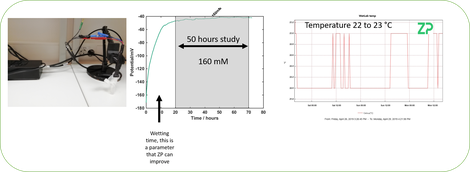
As we suspected that our data had been influenced by temperature we repeated the test but in a lab with a much better temperature control. The result was that after the initial setting time the signal was more stable so provides evidence that the sensor is temperature sensitive and so oen will either monitor or control the temperature when in use