Welcome to this week's newsletter from Zimmer and Peacock. This newsletter is a mixture of news, stories and tech notes from Zimmer and Peacock. If you want to subscribe to our newsletter or have any questions regarding Zimmer and Peacock and our passion for biosensor technologies please don't hesitate to contact us.
What every engineer should do when developing an electrochemically based sensor system
At Zimmer and Peacock we help our clients in the development and manufacturing of medical diagnostics and biosensors for their applications, programmes and products.
Sometimes the client is trying to develop both the reader electronics and the sensor in parallel, which can lead to problems when there is an issue with the sensors and/or the electronics; in this situation the client is left wondering 'is it my electronics, or is it my sensor?' A useful strategy when developing an electrochemical biosensor or medical diagnostic is to decouple the electronics development from the biosensor development, as too many dependencies between the two programmes can lead to confusion.
Zimmer and Peacock help their clients during the electrochemical sensor development by providing validation sensors. Our validation sensors have exactly the same form factor as our standard sensors and will give a signal output that is equivalent to the biosensor/sensor/medical diagnostic except the device is entirely solid state, i.e the device has a number of capacitors, resistors, diodes etc, in a parallel and/or series circuit which will give a response which is equivalent to the sensor under development. Find out more on our website here.
High impedance biosensors
This week Zimmer and Peacock had an enquiry as to whether the Ana Pot EIS was able to be used in biosensor applications, where the impedance was in the range 50 MOhm.
Our answer to the client was yes, but to help the client our team made up a validation sensor that was 56 MOhm, with a 0.1 nF capacitor in parallel with the resistor to give an imaginary component. Find out more here.
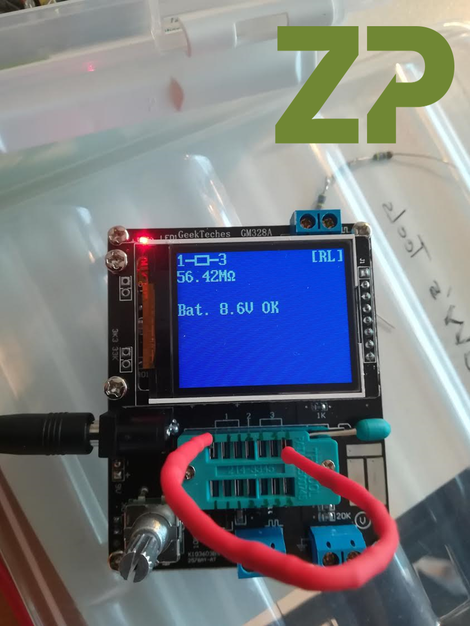
Choosing a threshold value for POC Diagnostics
Zimmer and Peacock support algorithm development for POC technologies for our clients. Part of the process is deciding on a threshold value for the POCT so that patients can be separated into groups with and without the disease of interest.
An example would be a sepsis point-of-care test (POCT) where the results are used for screening and separating patients with sepsis from those without so that treatment can be appropriately applied.
With any diagnostic there is a distribution in values for both the positive or negative results due to both the inherent variability with the technology but also within the patient groups, as everyone is unique. The question is how to choose a threshold value so that the algorithm can efficiently separate patients with sepsis from patients without, whilst maximising selectivity to avoid false positives and false negatives. Visit our website to find out more.